GlaxoSmithKline
Pharmaceutical Copywriting
A new sterile filling line required user instruction documents to be in place for training and GMP (Good Manufacturing Practice).
GSK required a couple of pharmaceutical copywriting services:
Technical Documentation
Every process used in pharmaceutical production is detailed in a flow chart called a Standard Operating Procedure (SOP). Each procedure offers a pictorial overview along with a summary and links to every SWI involved in that particular procedure.
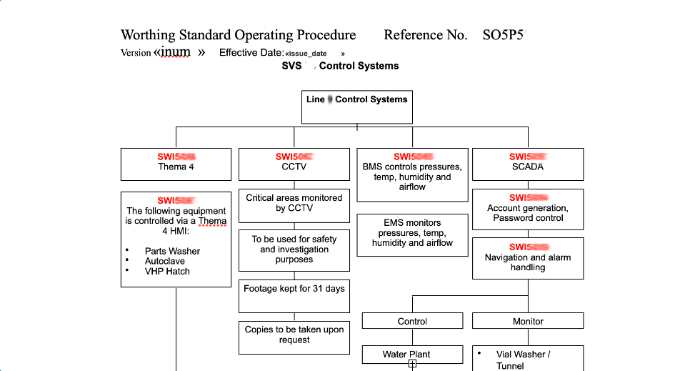
User Instructions
Every part of an SOP refers to a Standard Work Instruction (SWI) which provides step-by-step instructions on how to complete a certain task or process.
The SWI's were written with brief instructions for each step so they could easily be read by operators and accompanied by photos for nearly every stage. The SWI's were written by liaising with operators, SME's, equipment suppliers, technical support, engineers and QA. The finished draft was then user tested with operators, rewritten if required, before going through the approval process to get released.

Project Review
This multi-year project was completed when I was an employee of GSK in Worthing during the implementation of a new sterile filling line.
Originally, I was an aseptic area filling operator but then moved into the project team to help the new facility get online. This included writing and managing the completion of these pharmaceutical technical documents and getting them through the rigorous approval process in time.
My experience also extended to writing and completing other pharmaceutical technical documents such as logbooks, proforma's, Ways of Working (WOW) and training evaluations. This all involved liaising with multiple stakeholders as well as having an indepth knowledge of the line's processes and systems such as PMS, EMS, VHP, SCADA, sterile filling, and with company wide policies such as GMP, as well as industry standards such as The Orange Guide.


